c2h2 electron dot structure|Iba pa : Tagatay In the Lewis structure of C2H2 structure there are a total of 10 valence electrons. C2H2 is also called Acetylene (Ethyne). ---- Steps to Write Lewis Structure for compounds like C2H2. היכנסו לעמוד הרשמי של האח הגדול והתעדכנו בכל החדשות מהבית, שידור חי מבית האח הגדול, הצבעות בלייב, עדכונים בזמן אמת מהבית הכי מפורסם במדינה וצפייה ישירה בפרקים מלאים של האח הגדול
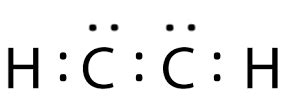
c2h2 electron dot structure,285K views 11 years ago. .more. A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure (Ethyne or Acetylene).For the C2H2 structure use the periodic table to find the. In the Lewis structure of C2H2 structure there are a total of 10 valence electrons. C2H2 is also called Acetylene (Ethyne). ---- Steps to Write Lewis Structure for compounds like C2H2. There are ten valence electrons in C2H2 molecule. C2H2 Lewis Structure. Lewis Structure of any molecule helps to know the arrangement of all atoms, their valence electrons, and the bond .For C 2 H 2 you have a total of 10 valence electrons to work with. In drawing the Lewis structure for C 2 H 2 (also called ethyne) you'll find that you don't have enough valence .The Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding between atoms in a molecule. .c2h2 electron dot structure Iba paC2H2 Lewis Structure - How to draw the Electron Dot Structure for Ethene | Success in Chemistry. Drawing the Lewis Structure for C 2 H 2 - Ethyne or Acetylene. Viewing . Lewis Dot Structure of C2H2 or CHCH (Acetylene or ethyne) - YouTube. kentchemistry.com. 25.1K subscribers. 299. 176K views 12 years ago Every Video. I quickly take you through how to draw the.
Mark charges on atoms if there are charges. Check the stability and minimize charges on atoms by converting lone pairs to bonds to obtain best lewis structure. Total number of electrons of the valance shells of C 2 . Watch on. 6 Steps to Draw the Lewis Structure of C2H2. Step #1: Calculate the total number of valence electrons. Here, the given molecule is C2H2 (or ethyne or . The -2 charge means that there are 2 extra electrons. Total: 4 + (3 × 6) + 2 = 24 electrons. The final answer MUST have this number of electrons‼! Step 2) Attach the atoms to each other using single bonds .2. H. 2. (Acetylene | Ethyne) Lewis Structure. C 2 H 2 (acetylene or ethyne) contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with . The total number of valence electrons in the acetylene or ethyne (C2H2) Lewis dot structure is 10. The molecular geometry or shape of C 2 H 2 is identical to its ideal electron pair geometry i.e., linear. The . C 2 H 2 Lewis structure. C 2 H 2 (acetylene or ethyne) has two carbon atoms and two hydrogen atoms. In the C 2 H 2 Lewis structure, there is a triple bond between the two carbon atoms, and each carbon is attached with one hydrogen atom, and none of the atoms has a lone pair. How to Draw the Lewis Dot Structure for C2H2: .C 2 H 2 is the simplest alkyne chemical compound with the chemical name Acetylene. Acetylene is also called Ethyne or Narcylen or Vinylene. It is widely used as a chemical building block and as a fuel. In its pure form, it is unstable and is handled as a solution. It is an unsaturated compound the two carbon atoms in it are linked together with .Q 4. Draw the possible isomers of the compound with molecular formula C3H6O and also give their electron dot structures. [2 Marks] [Exemplar] [Electron Dot Structure] View Solution. Q 5. Which among the following is the correct Lewis dot structure of ethyne ( .
c2h2 electron dot structureLewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.In ethene, each hydrogen atom has one unpaired electron and each carbon is sp 2 hybridized with one electron each sp 2 orbital. The fourth electron is in the p orbital that will form the pi bond. The bond order for ethene is simply the number of bonds between each atom: the carbon-carbon bond has a bond order of two, and each carbon-hydrogen .
2. H. 2. (Acetylene | Ethyne) Lewis Structure. C 2 H 2 (acetylene or ethyne) contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds. There are no lone pairs on carbon or hydrogen atoms. In this tutorial, we are going to learn how to draw the .
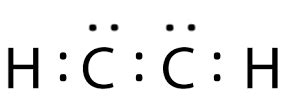
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and .Ethyne, also known as acetylene, is an organic chemical compound with the chemical formula C2H2. Since the entire chemical composition only features hydrogen and carbon atoms, this compound is a hydrocarbon. Ethyne is regarded by many to be the simplest alkyne since it consists of only two carbon atoms, which are triply bonded to each other.Q 5. Question 41. In electron dot structure, the valence shell electrons are represented by crosses or dots. (a) The atomic number is 17. Write its electronic configuration. (b) Draw the electron dot structure of chlorine molecule. View Solution.A step-by-step explanation of how to draw the C2H6 Lewis Dot Structure (Ethane).For the C2H6 structure use the periodic table to find the total number of val.Figure 7.10 Cations are formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed by atoms gaining electrons. The total number of electrons does not change. . To draw the Lewis structure for an odd-electron molecule like NO, we follow the same five steps we would for other molecules, but with a few minor .
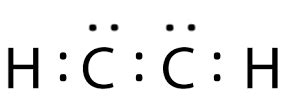
The Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding between atoms in a molecule. This structure is essential in understanding the properties and behavior of C2H2 in chemical reactions. C2H2 is a hydrocarbon compound made up of two carbon atoms and two .The Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding between atoms in a molecule. This structure is essential in understanding the properties and behavior of C2H2 in chemical reactions. C2H2 is a hydrocarbon compound made up of two carbon atoms and two .Iba paThe Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding between atoms in a molecule. This structure is essential in understanding the properties and behavior of C2H2 in chemical reactions. C2H2 is a hydrocarbon compound made up of two carbon atoms and two .GENERAL TERMS FOR LEWIS DOT STRUCTURES: 1. Dot • one dot represents one valence electron (found on odd-electron particles). 2. Pair of Dots •• a pair of dots represents a nonbonding (lone) pair of electrons that are not involved in a covalent bond and "belong to" only one atom. 3. Dash each dash represents two electrons that are .
The molecular formula of ethyne is C2H2. . To write the electron dot structure, put two hydrogen atoms at terminal positions and two carbon atoms in the internal positions. All the four atoms are placed in one line. Advertisement Advertisement siriraonavya siriraonavya Answer: the Lewis-dot structure the valance electrons are .
Write the electron-dot structures for : (i) ethane, (ii) ethene, and (iii) ethyne. View Solution. Q 2. Draw the electron-dot structure for ethyne. A mixture of ethyne and oxygen is burnt for welding. In your opinion, why cannot we use a mixture of ethyne and air for this purpose ?
c2h2 electron dot structure|Iba pa
PH0 · lewis structure of acetylene
PH1 · lewis dot structure for all elements
PH2 · lewis dot diagram maker
PH3 · electron dot structure for chlorine
PH4 · electron dot diagram for nitrogen
PH5 · electron dot diagram
PH6 · c2h2 electron geometry
PH7 · acetylene lewis dot structure
PH8 · Iba pa